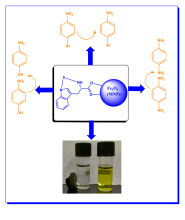
Sustainable magnetically recoverable Iridium coated Fe3O4 nanoparticles for enhanced catalytic reduction of organic pollutants in water
| Abstract | The reduction of nitroarenes to aromatic amines is one of the potential pathways to remediate the hazardous impact of toxic nitroarenes on the aquatic environment. Aromatic amines obtained from the reduction of nitroaromatics are not only less toxic than nitroaromatics but also act as important intermediates in the synthesis of dyes, drugs, pigments, herbicides, and polymers. There is a huge demand for the development of cost-effective, and eco-friendly catalysts for the efficient reduction of nitroarenes. In the present study, Fe3O4@trp@Ir nanoparticles were explored as efficient catalysts for the reduction of nitroarenes. Fe3O4@trp@Ir magnetic nanoparticles were fabricated by surface coating of Fe3O4 with tryptophan and iridium by co-precipitation method. As-prepared Fe3O4@trp@Ir nanoparticles are environmentally benign efficient catalysts for reducing organic pollutants such as 4-nitrophenol (4-NP), 4-nitroaniline (4-NA), and 1-bromo-4-nitrobenzene (1-B-4-NB). The key parameters that affect the catalytic activity like temperature, catalyst loading, and the concentration of reducing agent NaBH4 were optimized. The obtained results proved that Fe3O4@trp@Ir is an efficient catalyst for reducing nitroaromatics at ambient temperature with a minimal catalyst loading of 0.0025%. The complete conversion of 4-nitrophenol to 4-aminophenol took only 20 s with a minimal catalyst loading of 0.0025% and a rate constant of 0.0522 s−1. The high catalytic activity factor (1.040 s−1 mg−1) and high turnover frequency (9 min−1) obtained for Fe3O4@trp@Ir nanocatalyst highlight the possible synergistic effect of the two metals (Fe and Ir). The visible-light photocatalytic degradation of 4-NP was also investigated in the presence of Fe3O4@trp@Ir. The photocatalytic degradation of 4-NP by Fe3O4@trp@Ir is completed in 20 min with 95.15% efficiency, and the rate of photodegradation of 4-NP (0.1507 min−1) is about twice the degradation rate of 4-NP in the dark (0.0755 min−1). The catalyst was recycled and reused for five cycles without significant reduction in the conversion efficiency of the catalyst. |
| Faculty |
Harminder Kaur
|
|
hkaur@pec.edu.in
|
|
| More Information | DOI: 10.1007/s11356-023-26267-z |